
Does Hot Water Freeze Faster Than Cold? When you think about freezing water, the obvious assumption is that cold water should freeze faster than hot water. After all, it already starts closer to freezing point. But surprisingly, scientists and students alike have discovered cases where the opposite happens. This strange phenomenon is called the Mpemba Effect, and it’s one of the most fascinating puzzles in everyday science.
In this article, we’ll break down why this happens, what experiments reveal, and how you can use it to spark curiosity in learning science.(Does hot water freeze faster than cold?)
Does Hot Water Freeze Faster Than Cold?
The short answer: sometimes, yes. Under certain conditions, hot water can freeze faster than cold water. This unusual result has been observed for centuries and was famously documented by Erasto Mpemba, a Tanzanian student, in 1963; giving the effect its name.
The Mpemba Effect Explained
The Mpemba Effect refers to the counterintuitive observation that hot water can freeze faster than cold water. While it doesn’t always happen, when it does, scientists believe several factors may play a role:
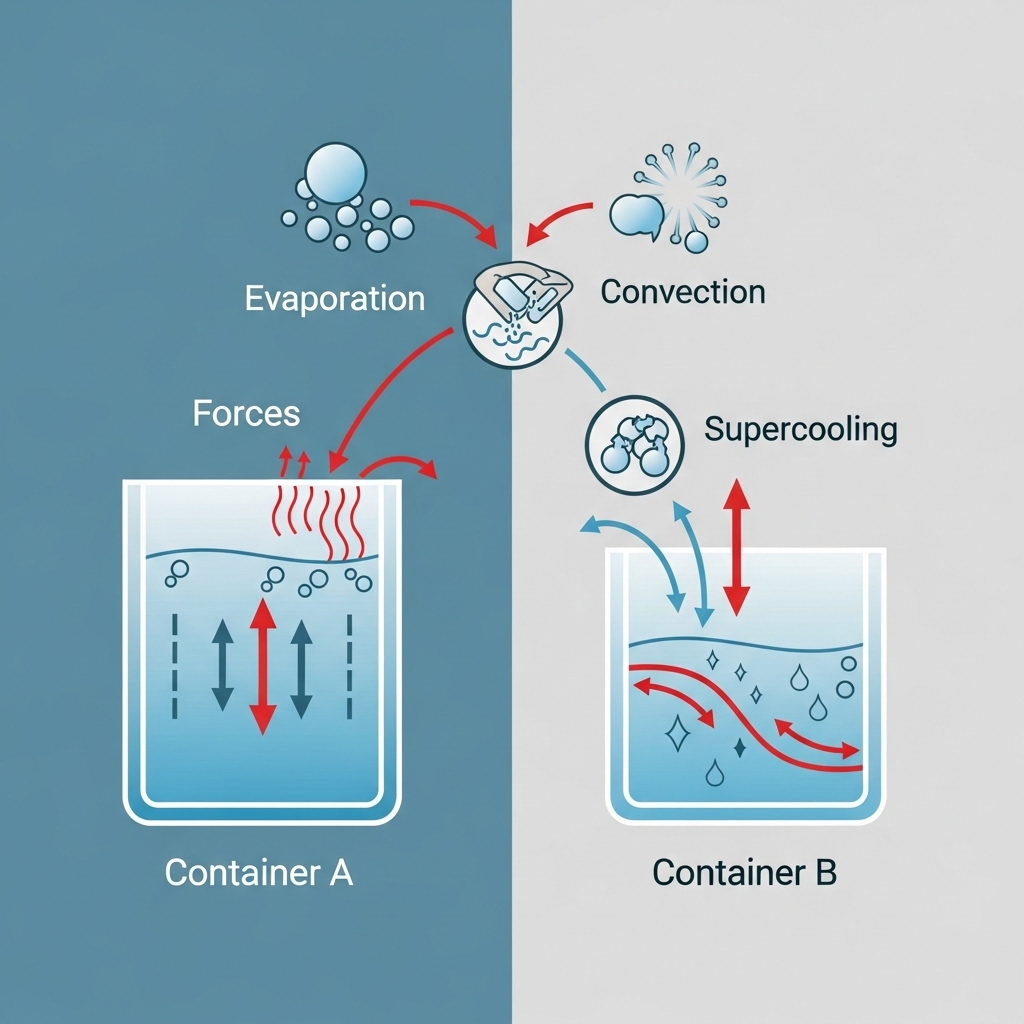
- Evaporation: Hot water loses mass as it evaporates, which means there’s less water left to freeze.
- Convection Currents: Hot water circulates differently as it cools, speeding up the cooling process.
- Supercooling: Cold water can sometimes avoid freezing below 0°C before crystallizing, while hot water “skips” this stage.
Real-Life Examples and Experiments
You don’t need a lab to test this, just a freezer, two identical containers, one filled with hot water and the other with cold water. Many science teachers use this as a classroom experiment to encourage observation and critical thinking.
🔍 Observation Tip: Sometimes the cold water still wins, but if the right conditions are in place (like container shape, temperature, and environment), the hot water can surprise you by freezing first.
Why Scientists Are Still Debating
Even though the Mpemba Effect has been studied for decades, there’s no single agreed explanation. Different studies highlight different factors, and the effect is not always reproducible. This ongoing mystery makes it one of the most exciting everyday science questions still under exploration.
The Role of Evaporation, Convection, and Supercooling
- Evaporation reduces the water volume, helping it freeze faster.
- Convection currents mix hot water more effectively during cooling.
- Supercooling delays freezing in cold water but less so in hot water.
What This Means for Students Learning Science
This question; Does hot water freeze faster than cold? – is a perfect example of curiosity-driven science learning. It shows that even simple, everyday things like water still have mysteries to uncover. For students, it encourages:
- Critical Thinking: Questioning assumptions.
- Experimentation: Testing theories hands-on.
- Curiosity: Understanding science beyond textbooks.
Teachers can use this phenomenon to inspire kids to run their own experiments, record results, and even debate conclusions.

The Fascinating Mystery of Freezing Water
So, does hot water freeze faster than cold? The fascinating answer is yes – but not always. The Mpemba Effect remains an open puzzle in science, showing that sometimes nature defies our expectations.
Next time you pour a hot drink or fill an ice tray, remember: even everyday water holds mysteries waiting to be explored.
Related Links:
Essential Skills for the Future Job Market in the United States
Back-to-School Tips and Essentials
Study Tips and Strategies for AIGP Exam Prep in the US
Why This Question Still Fascinates Us
The idea that hot water might freeze faster than cold water keeps sparking curiosity because it challenges what we expect from science. While researchers have proposed different explanations – from evaporation to molecular behavior — the truth is, it’s still partly a mystery. And that’s the beauty of science: even everyday questions, like what happens in your freezer, can lead to debates, experiments, and new discoveries.
So, next time you pour water into ice trays, try both hot and cold water and run your own mini-experiment. Who knows? You might notice something surprising that adds to the ongoing conversation!
Ready to take your learning to the next level?
At Educify.org, we bring you the best study tips, resources, and insights to help students grow smarter every day














2 Responses