
Does sugar boil water quicker? When you’re in the kitchen, waiting for water to boil can feel like forever. Some people swear that adding sugar makes the water boil quicker, while others argue it slows the process down. So, what’s the real science behind this everyday mystery? Let’s break it down in a fun, clear way that even students and curious minds can enjoy.(Does sugar boil water quicker?)
Sugar vs. Water: What Really Happens When You Boil Them
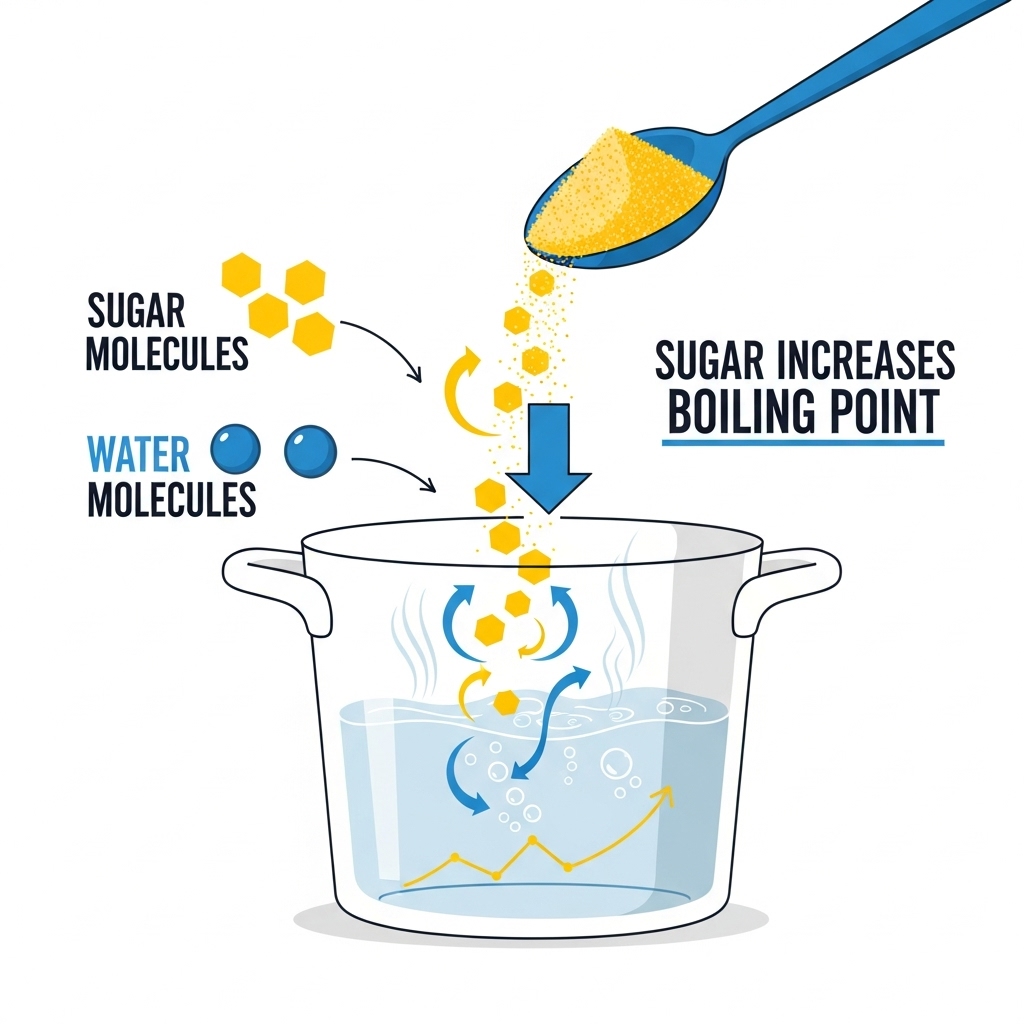
Here’s the deal: pure water boils at 100°C (212°F) at sea level. When you add sugar, you’re not just sweetening the liquid you’re changing its physical properties. (Does sugar boil water quicker?)
Sugar molecules mix with the water molecules, creating a solution. This interaction raises the boiling point of water. That means the water will actually need to reach a slightly higher temperature before boiling bubbles form.
In simple terms: sugar makes water boil slower, not faster. (Does sugar boil water quicker?)
The Science Behind It (Does sugar boil water quicker?)
The effect is called boiling point elevation, and it happens with any solute (sugar, salt, etc.) added to water. The dissolved particles interfere with the water molecules trying to escape into vapor. More heat energy is required to push them out, so the boiling point increases.
- With salt, the effect is stronger, which is why salty water boils hotter.
- With sugar, the effect exists but is slightly weaker. Still, it definitely does not speed up boiling.

Common Myths People Believe
- “Sugar boils faster because it heats up quickly.”
❌ Not true. While sugar can caramelize and darken when heated, it doesn’t mean the water itself reaches boiling sooner. - “The bubbles form faster with sugar, so it’s boiling quicker.”
❌ Those bubbles are often just air or dissolved gases escaping – not the true boiling point. - “It depends on how much sugar you use.”
✔️ This one has some truth. A tiny amount of sugar may not noticeably affect boiling. But the more sugar you add, the longer it will take.
Fun Kitchen Experiment You Can Try
Want to test it yourself? (Does sugar boil water quicker?) Grab these:
- 2 pots
- 2 cups of water each
- 2 tablespoons of sugar
- Boil one pot with plain water.
- Boil the second with water + sugar dissolved.
- Use a thermometer if you have one to see the difference.
You’ll notice the sugar water takes longer to bubble up – science in action!
Why This Matters Beyond the Kitchen
This isn’t just trivia – it’s applied science. Boiling point elevation is part of chemistry and physics lessons. It’s also useful in real life:
- Cooking & Baking: Knowing how sugar affects boiling can help with candy-making or syrups.
- Science Projects: It’s a simple but impressive experiment for school.
- Everyday Curiosity: Understanding why things happen makes us sharper thinkers.

Quick Recap
- Pure water boils at 100°C (212°F).
- Adding sugar raises the boiling point, making it take longer.
- Sugar does not make water boil faster – it slows it down.
- This is an example of boiling point elevation in action.
So, next time someone tells you sugar speeds things up, you can confidently say: “Nope – science proves otherwise!”

Related Reads You’ll Love
- Does Hot Water Freeze Faster Than Cold?
- Google vs. ChatGPT: Which One Helps Students Learn Better?
- Essential Skills for the Future Job Market in the United States
Sweet Truth Revealed 🍬
Adding sugar to water doesn’t speed up boiling – it slows it down by raising the boiling point. It’s a perfect example of how science hides in our daily lives, waiting for us to notice.
So the next time you’re cooking, remember: patience is key, and sugar is better saved for taste, not time.
Want more fun science answers like this? Visit Educify.org and keep your curiosity alive every day!














2 Responses